Supportive Care in Cancer
Official Journal of The Multinational Association of Supportive Care In Cancer (MASCC)
Published July 31, 2025
Study Summary: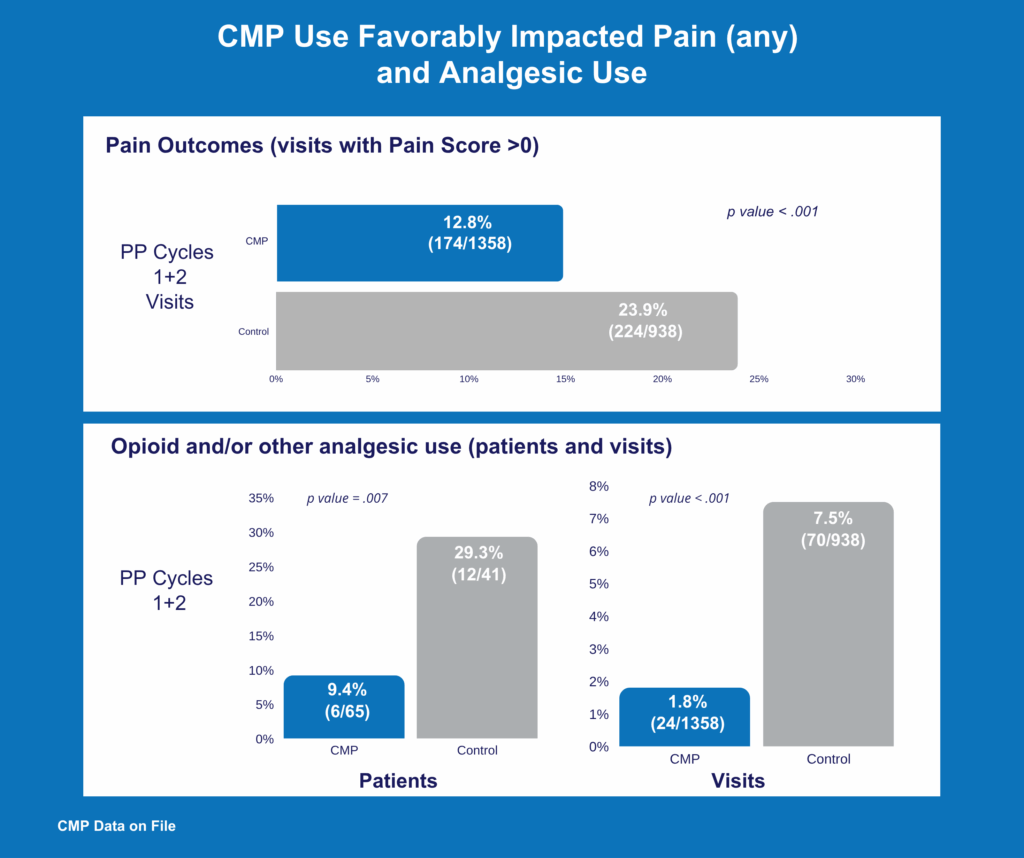 Results from a multi-center, randomized controlled trial demonstrated the Chemo Mouthpiece® significantly reduces the incidence of chemotherapy induced oral pain and lowers the need for opioids or analgesics, improving patient comfort and treatment adherence.
Results from a multi-center, randomized controlled trial demonstrated the Chemo Mouthpiece® significantly reduces the incidence of chemotherapy induced oral pain and lowers the need for opioids or analgesics, improving patient comfort and treatment adherence.
The trial evaluated the efficacy and tolerability of the Chemo Mouthpiece, a reusable oral cryotherapy device designed to help manage chemotherapy-induced oral mucositis (OM).
The study included 164 adult patients across 16 U.S. sites, randomized 2:1 to receive the Chemo Mouthpiece plus best supportive care (BSC) or BSC alone.
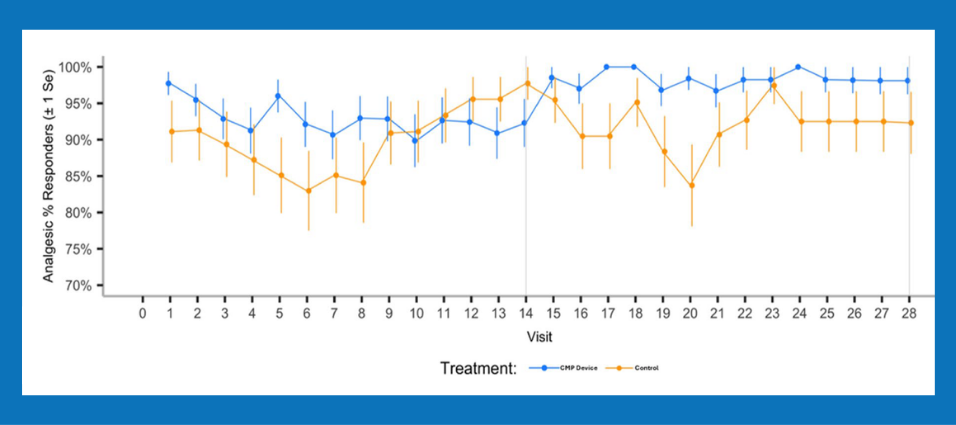
- Patients experienced less pain, used less analgesic pain meds, and used them for less time. Opioid pain medication use was eliminated in the Chemo Mouthpiece arm.
- Participants used the Chemo Mouthpiece starting 10 minutes before chemotherapy, throughout infusion, and at home at least twice daily for five days following infusion.
- Trial included regimens with short- and long-half-life chemotherapy agents.
- The Chemo Mouthpiece’s portability and ease of use enabled continued at-home use, which was associated with improved efficacy across regimens.
- Of 164 patients: more than 17 cancer types, over 30 different chemotherapies.
- No safety issues associated with use of the device.
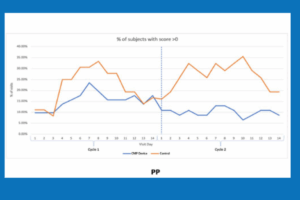
• 46% Reduction in oral pain episodes vs. control
• During peak symptom days (4–10), device users were pain-free 80% of the time vs. 68% with BSC
• 68% Reduction in opioid/analgesic use vs. control
• Use of the Chemo Mouthpiece eliminated opioid use in the per protocol population (Cycle 2)
• The device was effective, well-tolerated, and provided a convenient way to deliver consistent oral cryotherapy for patients at risk for oral mucositis.
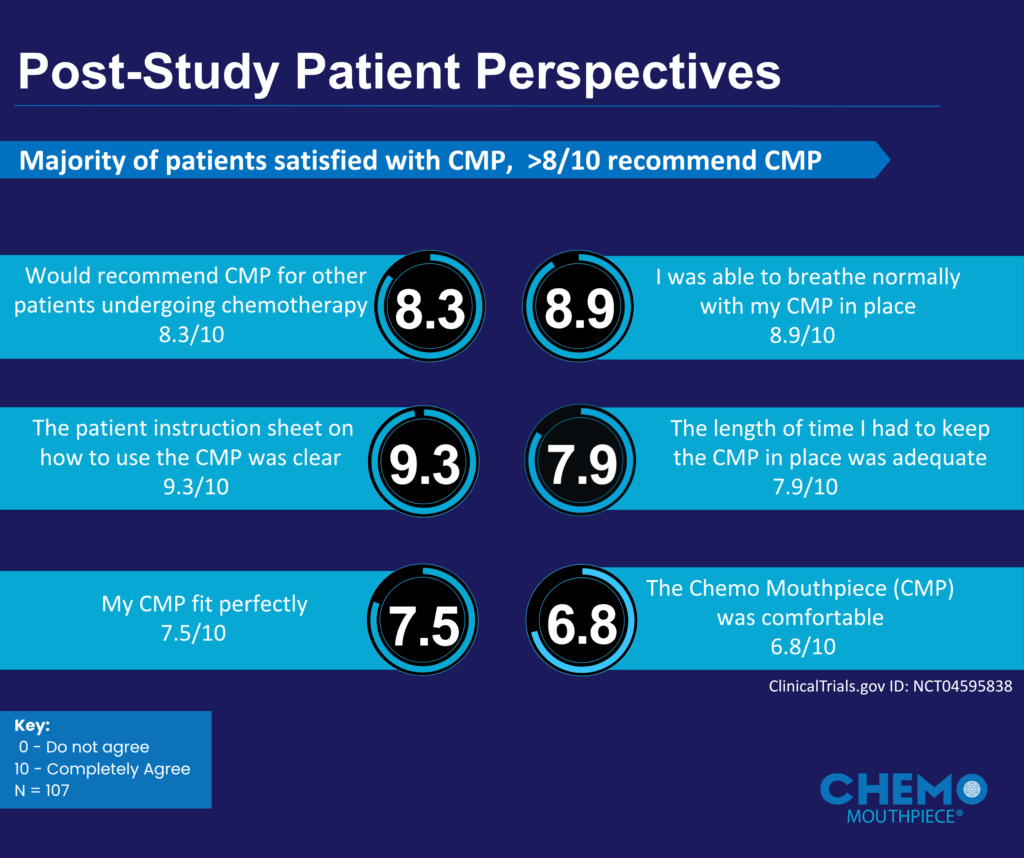
• 83% of patients would recommend the device to others undergoing chemo.
Read the full study in Supportive Care in Cancer:
Journal of Oncology Research and Therapy
Published: April 14, 2025
Study Summary:
A subset analysis of a multi-institutional, randomized controlled study shows that use of the Chemo Mouthpiece® significantly reduced pain and opioid/analgesic use among adult patients receiving chemotherapy for a broad range of cancers.
Key Study Findings:
• 68% reduction in all forms of analgesic use (Chemo Mouthpiece vs. control in the per protocol population).
• 76% reduction in the number of days patients reported using any analgesics for pain (Chemo Mouthpiece vs. control in the per protocol population).
• Use of the Chemo Mouthpiece eliminated opioid use in the per protocol population.
The study provides strong evidence that the Chemo Mouthpiece effectively reduced the need for all forms of analgesics to manage chemotherapy induced oral mucositis associated pain.
Read the full study in Journal of Oncology Research and Therapy: